|
Condition |
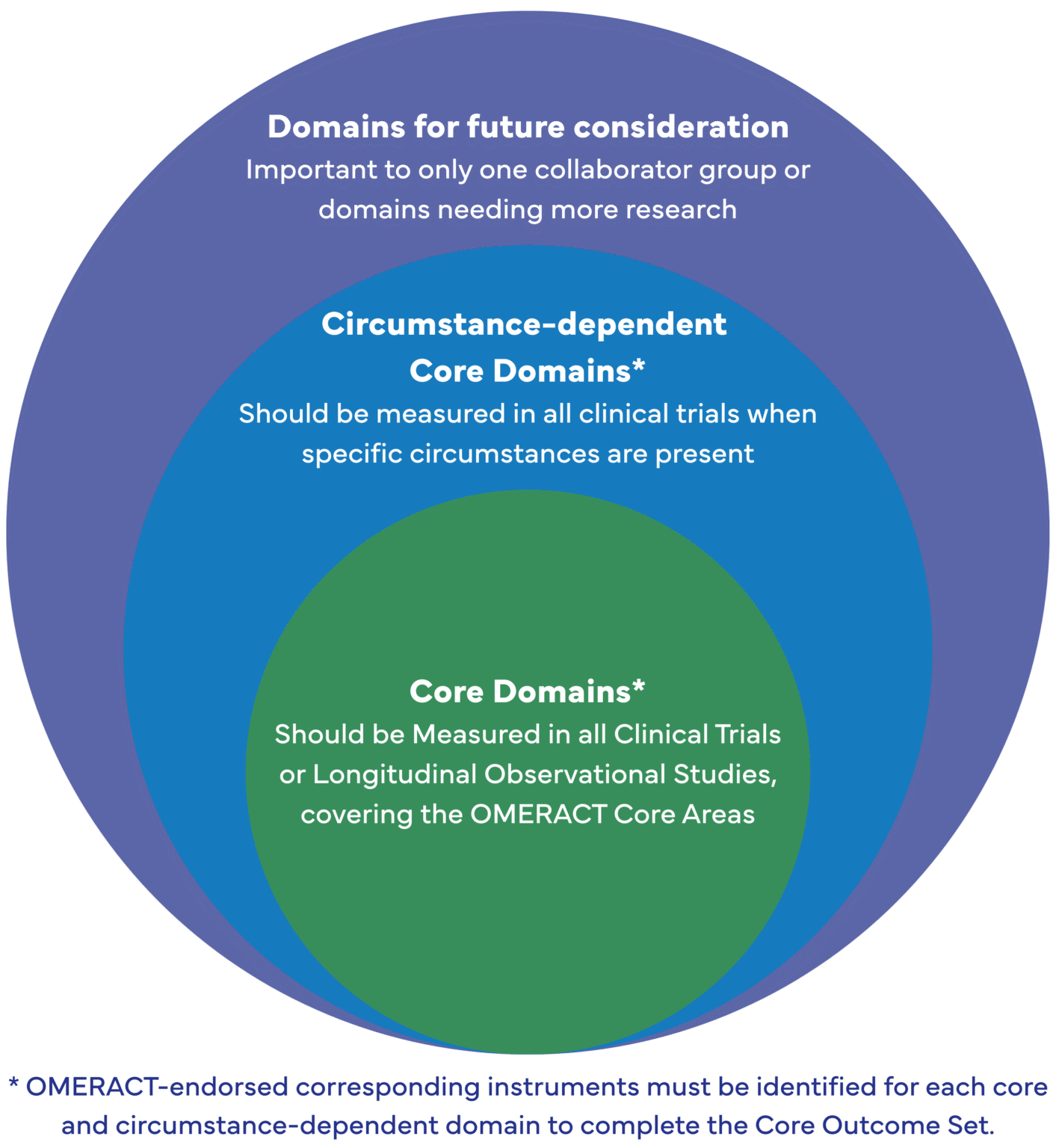
Core Domains |
Endorsed
Instruments |
Working Group
Page |
|
Joint Health Conditions |
|
OMERACT Core Domains for Musculoskeletal Health Research |
OMERACT Core Domains for Musculoskeletal Health Research |
|
Visit WG Webpage |
|
Health-Related Quality of Life (HRQoL) |
|
|
Pain Intensity |
|
|
Pain
Interference |
|
|
Physical
Function |
|
|
Axial Spondyloarthritis |
Disease
Activity |
ASDAS
(Ankylosing Spondylitis Disease Activity Score) |
Visit WG Webpage |
|
Patient global assessment of disease activity (NRS) |
|
SPARCC MRI activity of the SIJ |
|
SPARCC MRI activity of the spine |
|
Fatigue |
NRS fatigue (BASDAI Q1) |
|
Morning Stiffness |
Severity and duration of stiffness (BASDAI (Q5 +Q6)/2) |
|
Overall Functioning & Health |
ASAS-HI |
|
Pain |
NRS total back pain (BASDAI Q2) |
|
Physical Functioning |
BASFI |
|
Adverse Events including Death |
|
|
Circumstance-dependent Core Domains: |
|
Extra-musculoskeletal manifestations |
AAU |
|
IBD |
|
Psoriasis |
|
Peripheral manifestations |
44 swollen joint count |
|
Dactylitis count (including active fingers and/or toes) |
|
MASES |
|
Structural Damage |
mSASSS |
|
Gout - Acute |
Activity Limitations |
|
Visit WG Webpage |
|
Joint Swelling |
4-point Likert Scale |
|
Joint Tenderness |
4-point Likert Scale |
|
Pain |
5-point Likert Sale or VAS |
|
Patient Global Assessment |
5-point Likert Scale |
|
Gout - Chronic |
Acute Gout Attack |
|
|
Activity Limitations |
|
|
Health-Related Quality of Life (HRQoL) |
|
|
Pain |
|
|
Patient Global Assessment |
|
|
Serum Urate |
|
|
Tophus Burden |
|
|
Hand Osteoarthritis (OA) |
Hand Strength |
|
Visit WG Webpage |
|
Health-Related Quality of Life
(HRQoL) |
|
|
Joint activity |
|
|
Pain |
|
|
Patient Global Assessment |
|
|
Physical Function |
AUSCAN |
|
MHQ |
|
Hip & Knee Osteoarthritis (OA) |
Joint Structure |
|
Visit WG Webpage |
|
Pain |
|
|
Patient Global
Assessment |
|
|
Physical Function |
|
|
Health-Related
Quality of Life (HRQoL) |
|
|
Adverse events
including death |
|
|
Juvenile Idiopathic Arthritis (JIA) |
Activity limitations/physical function |
|
Visit WG Webpage |
|
Joint inflammatory
signs |
|
|
Pain |
|
|
Patient perception of overall well-being related to
disease
•Overall well-being
•Disease activity |
|
|
Adverse events
including death |
|
|
Polymyalgia Rheumatica (PMR) |
Pain |
|
Visit WG Webpage |
|
Physical Function |
|
|
Stiffness |
|
|
Systemic
Inflammation (laboratory blood tests) |
|
|
Adverse Events
including Death |
|
|
Psoriatic Arthritis (PsA) |
Fatigue |
|
Visit WG Webpage |
|
HRQOL |
PsAIS12 |
|
MSK Disease
Activity |
66/68 Joint Count |
|
Pain |
|
|
Physical
function |
HAQ-DI |
|
SF-36 |
|
Physician’s
Global |
|
|
Skin Disease
Activity |
|
|
Systemic
Inflammation |
|
|
Rheumatoid
Arthritis |
Acute
phase reactants |
|
Visit WG Webpage |
|
Fatigue |
|
|
Pain |
|
|
Patient global
assessment |
|
|
Physical
disability |
|
|
Physician
global assessment |
|
|
Radiographs of
joints (In studies of 1 or more years’ duration) |
|
|
Swollen joints |
|
|
Tender joints |
|
|
Shoulder Disorders |
Pain |
|
Visit WG Webpage |
|
Patient global-shoulder |
|
|
Physical
function/activity; |
|
|
Adverse Events
including Death |
|
|
Systemic Rheumatologic Health Conditions |
|
ANCA-Associated
Vasculitis |
Disease
Activity |
|
Visit WG Webpage |
|
Health-Related Quality of Life (HRQoL) |
|
|
Organ Damage Assessment |
|
|
Adverse
Events including Death |
|
|
Behçet’s Syndrome |
Disease
Activity |
|
Visit WG Webpage |
|
Health-Related Quality of Life (HRQoL) |
|
|
New Organ Involvement |
|
|
Adverse
Events including Death |
|
|
Circumstance-dependent Core Domains: |
|
Mucocutaneous |
|
Number of lesions |
|
|
Pain of lesions |
|
|
Central Nervous
System |
|
CNS Lesion |
|
|
Cognitive Function |
|
|
Neurologic Function |
|
|
Gastrointestinal |
|
Clinical GI Activity |
|
|
Endoscopic GI Activity |
|
|
Vascular |
|
Vascular Lesion |
|
|
Thrombophlebitis |
|
|
Post-Thrombotic Syndrome |
|
|
Musculoskeletal |
|
Tender Joints |
|
|
Swollen Joints |
|
|
Eye |
|
Visual Acuity |
|
|
Frequency of Attacks |
|
|
Ocular Severity |
|
|
Vascular Leakage |
|
|
CTD-ILD |
Cough |
|
Visit WG Webpage |
|
Dyspnea |
|
|
Functional Status |
|
|
HRQOL |
|
|
Lung Imaging |
|
|
Lung Physiology |
Forced Vital Capacity |
|
Adverse Events including Death |
|
|
Fibromyalgia |
Fatigue |
|
Visit WG Webpage |
|
Function |
|
|
Joint Tenderness |
|
|
Multidimensional |
|
|
Pain |
|
|
Patient Global Assessment |
|
|
Sleep Disturbance |
|
|
Large
Vessel Vasculitis |
Arterial
Function |
|
Visit WG Webpage |
|
Biomarkers |
|
|
Fatigue |
|
|
Organ Function |
|
|
Pain |
|
|
Adverse events including death |
|
|
Myositis |
Fatigue |
|
Visit WG Webpage |
|
Pain |
|
|
Physical Function |
|
|
Adverse events including death |
|
|
Osteoporosis |
Musculoskeletal Signs Of Fractures/Height |
|
Visit WG Webpage |
|
Biomarkers:
Fractures (Imaging) , Bone Metabolism (Soluble) , Bone Mineral Density
(Other) |
|
|
Systemic Sclerosis - Raynaud’s Phenomenon |
Duration Of Attacks Of RP |
|
Visit WG Webpage |
|
Frequency Of Attacks Of RP |
|
|
Health-Related Quality Of Life
(HRQoL) |
|
|
Hospitalization Or Need For Urgent
Intervention For RP |
|
|
Impact Of RP On Daily
Life/Adaptation |
|
|
Impact Of RP On Function |
|
|
Pain Intensity Due To RP |
|
|
Severity Of Attacks Of Rp |
|
|
Adverse Events, Including Death |
|
|
Systemic Sclerosis - Digital Ulcers (DUs) |
Global Burden Of DUS |
|
|
Healing Of DUS |
|
|
Health-Related Quality of Life
(HRQoL) |
|
|
Hospitalization Or Need For Urgent
Intervention For DUS |
|
|
Impact Of DUS On Daily
Life/Adaptation |
|
|
Impact Of DUS On Function |
|
|
Number Of DUS |
|
|
Pain Intensity Due To DUS |
|
|
Physician Global Assessment Of DUS |
|
|
Adverse Events, Including Death |
|
|
Systemic
Lupus Erthematosus
(currently being updated) |
Disease Activity |
|
Visit WG Webpage |
|
Health-Related Quality Of Life (Hrqol) |
|
|
Organ Damage |
|
|
Adverse Events, Including Death |
|