About the Late-stage Knee and Hip Osteoarthritis Composite Measure Working Group
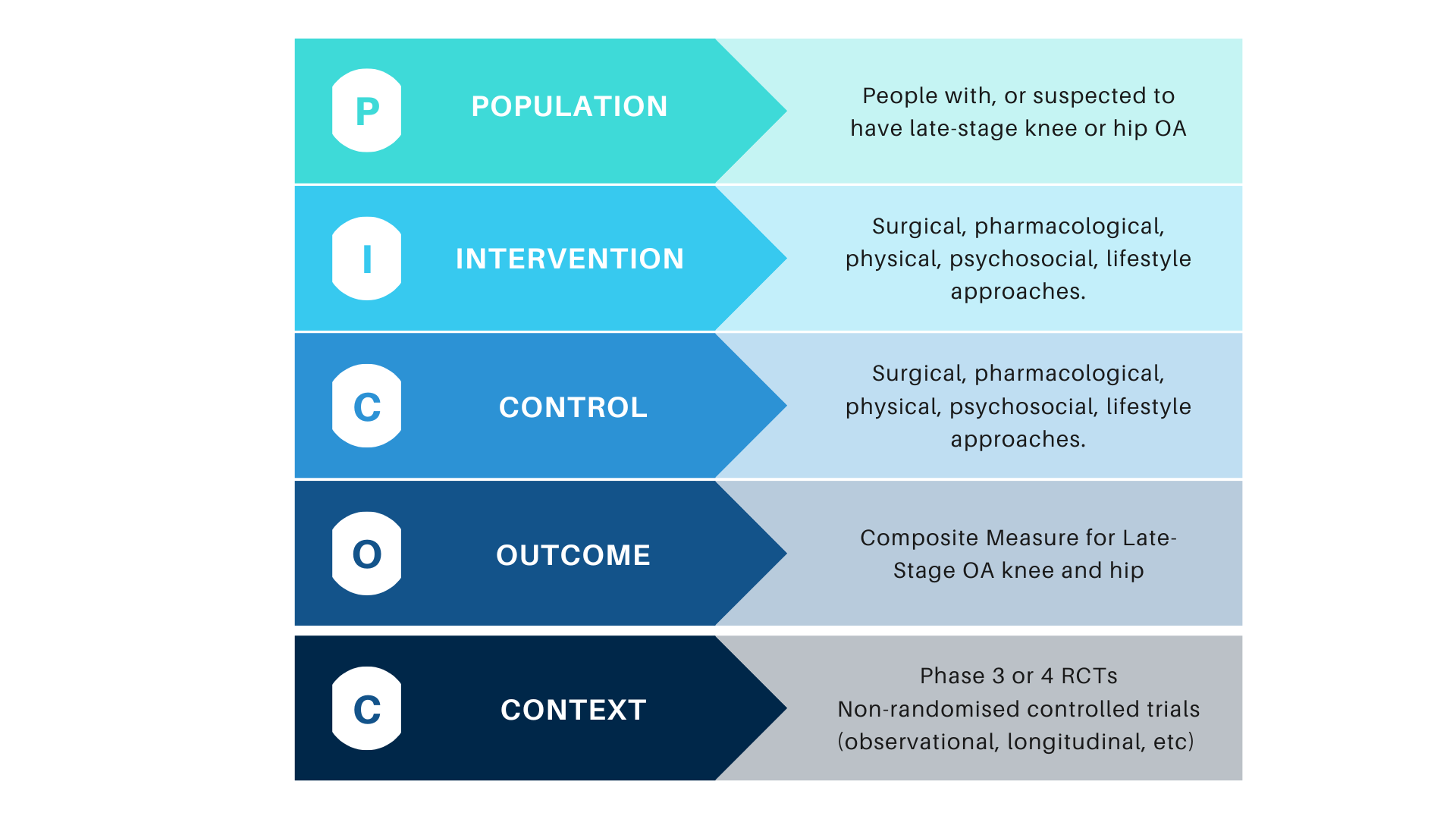
The receipt of a total knee (TKR) or hip replacement (THR) has commonly been used in clinical trials as an outcome measure of “late-stage” osteoarthritis (OA). However, total joint replacement outcomes are highly variable, dependent on a patient’s access and preference for surgery, the surgeon’s preference, socio-economic and geographical factors. There have been calls by the clinical trial community and clinical regulators to define a clinical endpoint for “late stage OA”, leading to the development of a new composite measure that can be used as both an outcome measure or inclusion/exclusion criteria in clinical trials.
The aims of this working group are to generate and define a set of candidate target domains to be included in all studies that aim to prevent or reduce TKR or THR, with a view to future developing a composite measure of the late-stage OA.

Francis Guillemin
Co-Chair

Gillian Hawker
Co-Chair

David Hunter
Co-Chair

Lyn March
Co-Chair

Jocelyn Bowden
Fellow
WORKING GROUP PUBLICATIONS
Working Group Members:
Adenike Adebajo
Adewale Adebajo
Ali Mobasheri
Angie Botto-van Bemden
Aya Akmal Amin
Bev Shea
David Felson
David Hunter
Denise Bury
Esmarie Venier
Fernando Pimentel-Santos
Francis Guillemin
Gillian Hawker
Illana Ackerman
Jane Stretton
Javier Rios
Jean-Noel Talabardon
Jocelyn Bowden
Jurgen Loffler
Liliia Shvets
Lyn March
M Masudul Hassan
Madusha Menu Cristeen Jayasinghe
Marie March
Motahareh Karimijashni
Oliver Krâmer
Olufemi Adelowo
Panagiotis Ermeidis
Peter Tugwell
Philip Conaghan
Philippa Nicolson
Qiuke Wang
Rashed Toticell
Richard Loeser
Sevdalina Lambova
Sougata Panda
Tamer Gheita
Tracy Stryczynski
Win Min Oo
Yari Nickolic