About the Contextual Factors Working Group
In 2012, ‘contextual factors’ was introduced for the first time in the OMERACT process, but identifying, understanding and approaching contextual factors proved difficult. The Contextual Factors Working Group (CFWG) was formed to provide guidance on how to address these challenges. An essential part of the research plan includes developing an operational definition and guidance on how to address contextual factors in rheumatology trials, when developing core outcome measurement sets

Robin Christensen
Co-Chair

Lyn March
Co-Chair

Peter Tugwell
Co-Chair

Farwa Asim
Fellow

Midhat Kamal
Fellow

Max Mischkewitz
Fellow

Niti Goel
Patient Research Partner

Amye Leong
Patient Research Partner
OMERACT consensus-based operational definition of contextual factors:
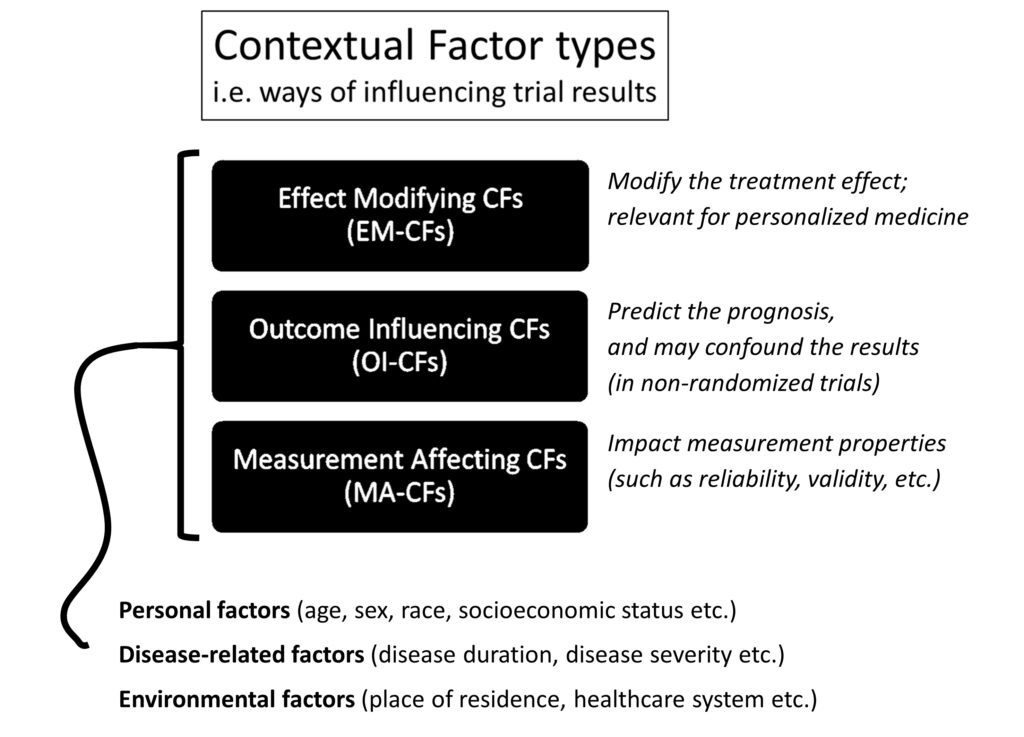
Overview of the consensus-based operational definition of contextual factors. The three contextual factor types describe different ways that contextual factors can influence the results of a trial. Brief descriptions of each type are shown in the figure. All three types are described in detail in the material below. In short, EM-CFs modify the treatment effect (i.e. some patient subgroups experience greater or less effect from a treatment compared to other subgroups). OI-CFs are prognostic factors (sometimes called risk factors), i.e. factors predicting the course of a patient’s condition and may confound the results of trials that are not randomized. MA-CFs influence the performance of outcome measurement instruments (such as reliability, validity, responsiveness, etc.). To guide which specific factors could be considered contextual factors, the factors must fit within one of the three classification categories, i.e. either personal-, disease-related, or environmental factors. The contextual factor types are not mutually exclusive, so some specific factors, e.g. sex, may both be an EM-CF, OI-CF, and MA-CF. CFs, Contextual Factors. Figure published in Nielsen et al. Semin Arthritis Rheum. 2021 Jun;51(3):601-606.
About Contextual Factors Working Group
What are Contextual Factors?
Working Group Publications
(Nielsen et al. 2021) OMERACT consensus-based operational definition of contextual factors in rheumatology clinical trials: A mixed methods study
(Nielsen et al. 2020) Towards consensus in defining and handling contextual factors within rheumatology trials: an initial qualitative study from an OMERACT working group
(Nielsen et al. 2019) Identifying Provisional Generic Contextual Factor Domains for Clinical Trials in Rheumatology: Results from an OMERACT Initiative
(Finger et al. 2017) An OMERACT Initiative Toward Consensus to Identify and Characterize Candidate Contextual Factors: Report from the Contextual Factors Working Group
Working Group Members:
Aidan Cashin
Allyson Jones
Amye Leong
Angie Botto-van Bemden
Annelies Boonen
Anupam Wakhlu
Aya Akmal Amin
Ayano Kelly
Barney Reeves
Beverley Shea
Caroline Flurey
Catherine Hill
Catherine Hofstetter
Christie Bartels
Christoph Pohl
Clifton Bingham
Courage Uhunmwangho
Daniel Furst
Danielle van der Windt
Diana Hollander
Dinesh Khanna
Dorcas Beaton
Dorthe Berthelsen
Edith Brown
Ernest Choy
Esen Cam
Eva E. Waehrens
Farwa Asim
Francesca Ingegnoli
Francis Guillemin
George Wells
Gerd Jenny Aanerud
Graham Macdonald
Gulen Hatemi
Helene Storgaard
Ilfita Sahbudin
Irene van der Horst-Bruinsma
Ivana Silva
Jasvinder Singh
jennifer Petkovic
Joachim Musaus
Josef Smolen
Karina Torralba
Karine Toupin April
Kathleen Tymms
Lars Erik Kristensen
Laure Gossec
Lihi Eder
Linda Li
Lisa Christopher-Stine
Lisa Stamp
Lyn March
Maarten Boers
Maarten de Wit
Margreet Kloppenburg
Maria Angeles Lopez-Olivo
Maria Suarez-Almazor
Mary Cowern
Max Mischkewitz
Maya Desai
Michael G. Lyon
Michael Gill
Midhat Kamal
Monika Finger
Nataliya Milman
Niti Goel
Pamela Richards
Patricia Hurley
Peter Taylor
Peter Choong
Peter Merkel
Peter Tugwell
Philip Conaghan
Philip Mease
Polina Putrik
Prof. Tamer Gheita
Raouf Hajji
Rebecca Johnson
Reuben Escorpizo
Richard Vesely
Rieke Alten
Robin Christensen
Sabrina Mai Nielsen
Sarah Mackie
Sasikala Bheemireddy
Saurab Sharma
Shawna Grosskleg
Simon Stones
Sofia Ramiro
Stacey Grealis
Stanton Newman
Suzanne Verstappen
Thasia Woodworth
Thomas Castelnovo
Torkell Ellingsen
Vibeke Strand
Victor Sloan
Will Taylor
Nehal Nadeem