About the Shared Decision Making Working Group
Shared decision making (SDM) is central to patient-centered care and is at the crossroads between evidence-based medicine and patient-centered care. In the last decade, there has been increasing interest in SDM in rheumatology and an imperative to use SDM to achieve optimal care.
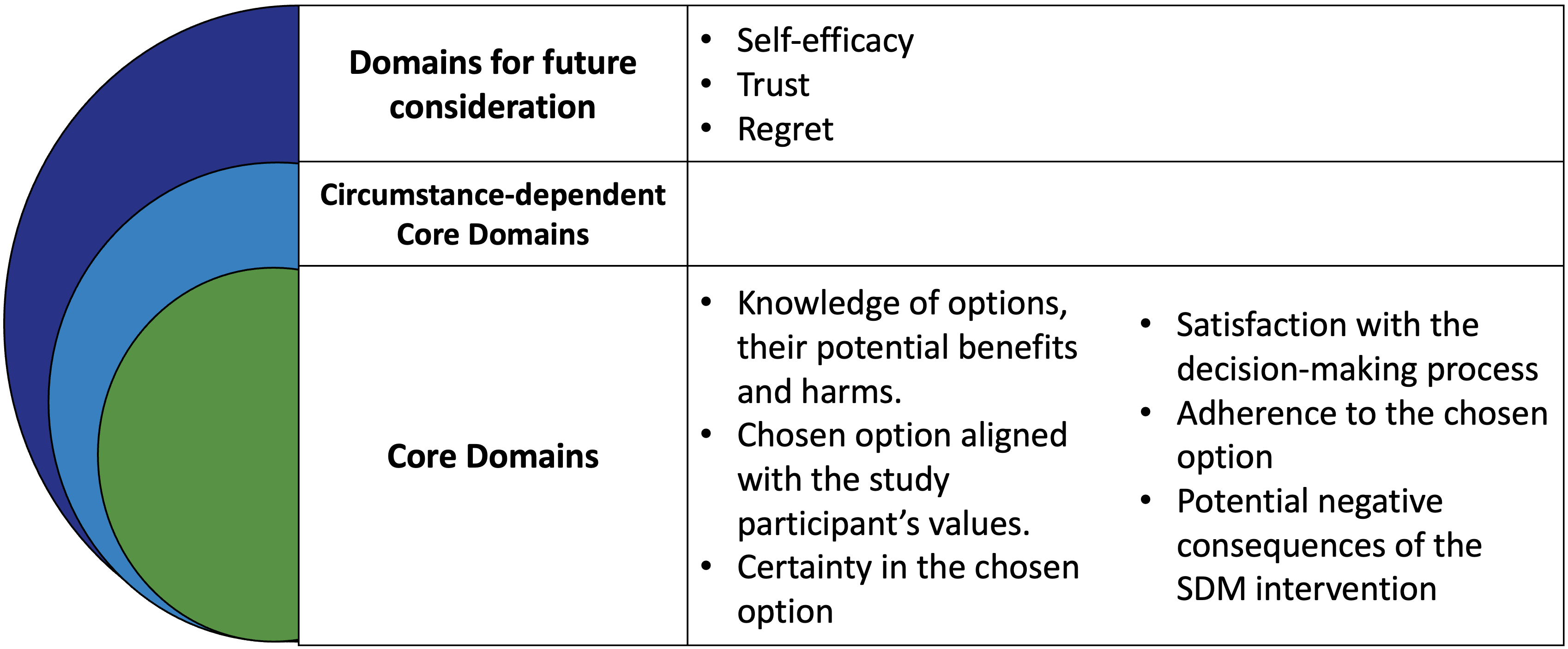
The use of consensus-building methods following the OMERACT Filter 2.1 methodology, grounded in a patient-oriented approach, led to strong endorsement of a core domain set for SDM interventions in rheumatology trials.
Future research will include the development of a core outcome measurement set to identify instruments to assess these domains in trials of SDM interventions.
The OMERACT SDM WG is classified as a ‘bolton’ group. ‘Bolt-on groups’ describe the additional domains and instruments that are part of a specific intervention, and which are measured in addition to disease-specific core outcome sets. In a clinical trial of SDM interventions, the trial must measure both the core outcome set specific to the concept of SDM and include the disease-specific core outcome set of the clinical trial’s study population. By doing so, we ensure that we measure both intervention-specific and disease-specific outcomes.

Jennifer Barton
Co-Chair

Peter Brooks
Co-Chair

Simon Décary
Co-Chair

Liana Fraenkel
Co-Chair

Linda Li
Co-Chair

Alexa Meara
Co-Chair

Karine Toupin April
Co-Chair
What is shared decision making?
What are shared decision making outcomes?
OMERACT Endorsed Bolt On for Clinical Trials of SDM interventions
RECENT WORKING GROUP PUBLICATIONS
OMERACT Core outcome measurement set for shared decision making in rheumatic and musculoskeletal conditions: a scoping review to identify candidate instruments.
Consensus on the definitions and descriptions of the domains of the OMERACT Core Outcome Set for shared decision making interventions in rheumatology trials.
Endorsement of the OMERACT core domain set for shared decision making interventions in rheumatology trials: Results from a multi-stepped consensus-building approach
Development of a core domain set for shared decision making interventions in rheumatology: An OMERACT white paper with stakeholders’ input
Development of a Draft Core Set of Domains for Measuring Shared Decision-Making in Osteoarthritis: An OMERACT Working Group on Shared Decision Making
Toward the Development of a Core Set of Outcome Domains to Assess Shared Decision-making Interventions in Rheumatology: Results from an OMERACT Delphi Survey and Consensus Meeting
Development of a Draft Core Set of Domains for Measuring Shared Decision Making in Osteoarthritis: An OMERACT Working Group on Shared Decision Making