OMERACT Rheumatoid Arthritis (RA) Core Outcome Set
What Is It?
The OMERACT Rheumatoid Arthritis (RA) Core Outcome Set is a consensus-driven, internationally endorsed set of outcome domains that should be measured in all clinical trials evaluating treatments for RA. Originally developed through collaboration with the World Health Organization (WHO) and the International League of Associations for Rheumatology (ILAR), the core set was established in 1994 and has since become a cornerstone of standardized outcome measurement in rheumatology research.
Why Was It Developed?
Before the introduction of the core set, clinical trials in RA varied widely in how they measured outcomes. This inconsistency made it difficult to compare treatments or interpret evidence across studies. Some outcomes were insensitive to change or duplicated others, and many failed to reflect what matters most to patients.
OMERACT brought together global experts—including rheumatologists, methodologists, regulators, and patients—to agree on a minimum set of outcomes that are:
-
Clinically relevant
-
Patient-centered
-
Responsive to change
-
Feasible for trials worldwide
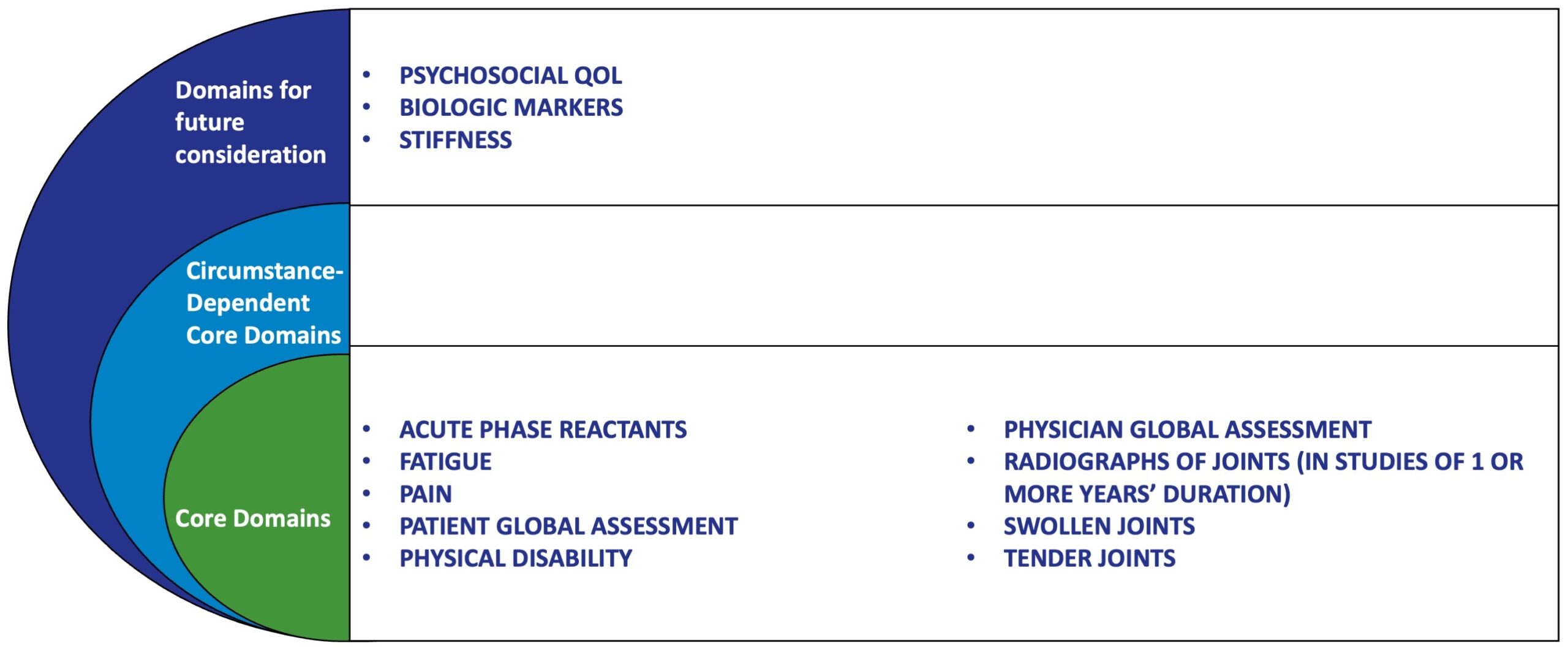
What’s in the RA Core Set?
The original RA Core Set includes 7 mandatory outcome domains to be measured in all trials of symptom-modifying antirheumatic drugs (SMARDs), plus an additional imaging measure for trials lasting ≥1 year. Since its introduction, the RA Core Set has undergone evaluation and refinement. At OMERACT 8, fatigue—long overlooked in clinical trials but frequently reported by patients—was formally recommended as an additional patient-centered outcome measure to be included wherever feasible.