About the Polymyalgia Rheumatica Working Group
Polymyalgia rheumatica (PMR) is an inflammatory disease characterized by subacute onset pain and stiffness in the shoulders and hips. Oral glucocorticoids represent the mainstay of treatment and while cessation of therapy is the ultimate goal, up to 50% of patients with PMR continue to require prednisolone 2–3 years after diagnosis.
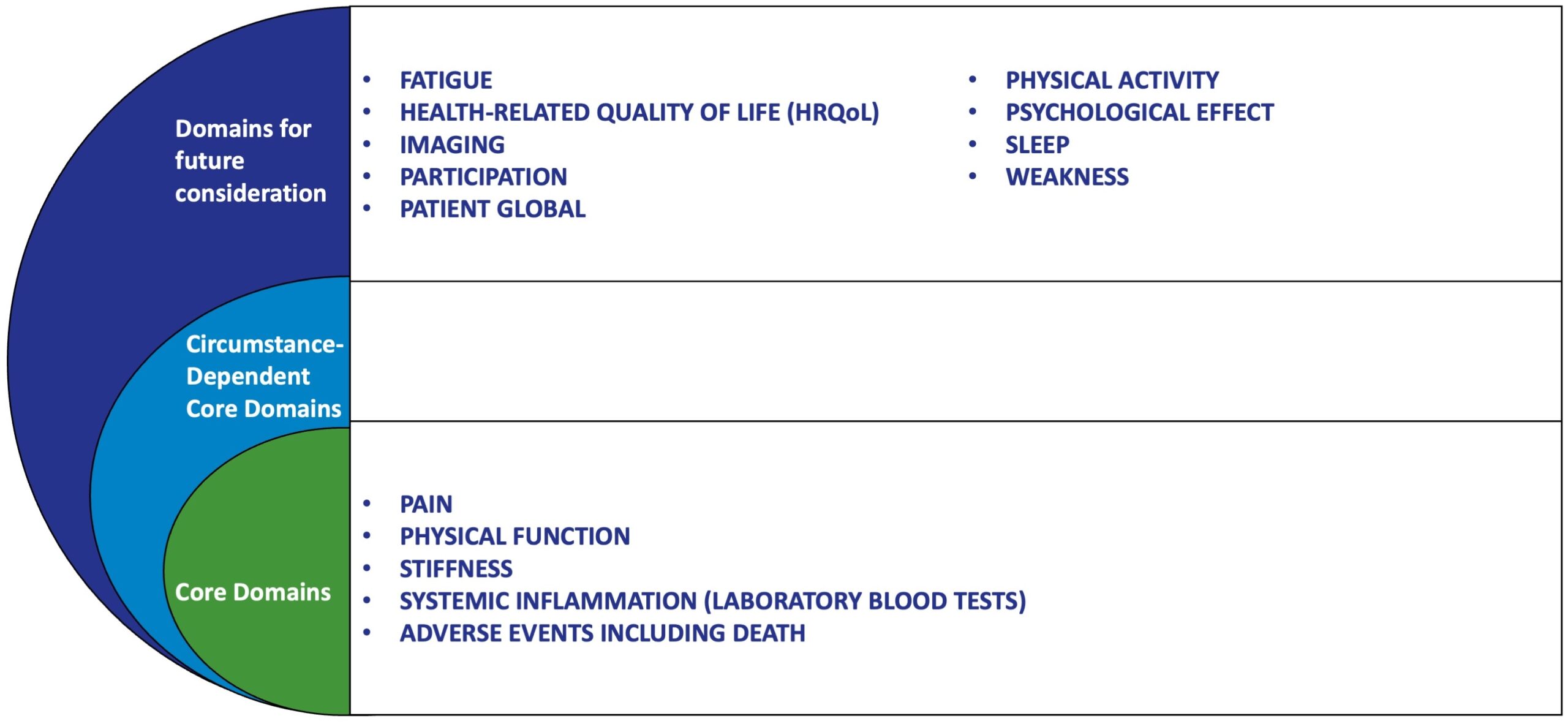
In 2016, the Outcome Measures in Rheumatology (OMERACT) endorsed a core domain set for PMR. The inner core of the “onion,” signifying items to be measured in all PMR clinical trials, consisted of 4 domains: pain, stiffness, physical function, and systemic inflammation.
Thomas Bolhuis
Co-Chair

Catherine Hill
Co-Chair

Sarah Mackie
Co-Chair

Claire Owen
Co-Chair

Sebastian Sattui
Co-Chair

Lee Simon
Co-Chair

Max Yates
Co-Chair
Sharon Cowley
Fellow
Raisa Lomanto Silva
Fellow
Task Toyoda
Fellow
Victor Yang
Fellow
OMERACT Endorsed Core Domain Set for Polymyalgia Rheumatica (PMR)
RECENT WORKING GROUP PUBLICATIONS
Test-Retest Reliability of Pain VAS/NRS, Stiffness VAS/NRS, HAQ-DI and mHAQ in Polymyalgia Rheumatica: An OMERACT Study
Toward a Core Outcome Measurement Set for Polymyalgia Rheumatica: Report from the OMERACT 2018 Special Interest Group
The OMERACT Core Domain Set for Outcome Measures for Clinical Trials in Polymyalgia Rheumatica