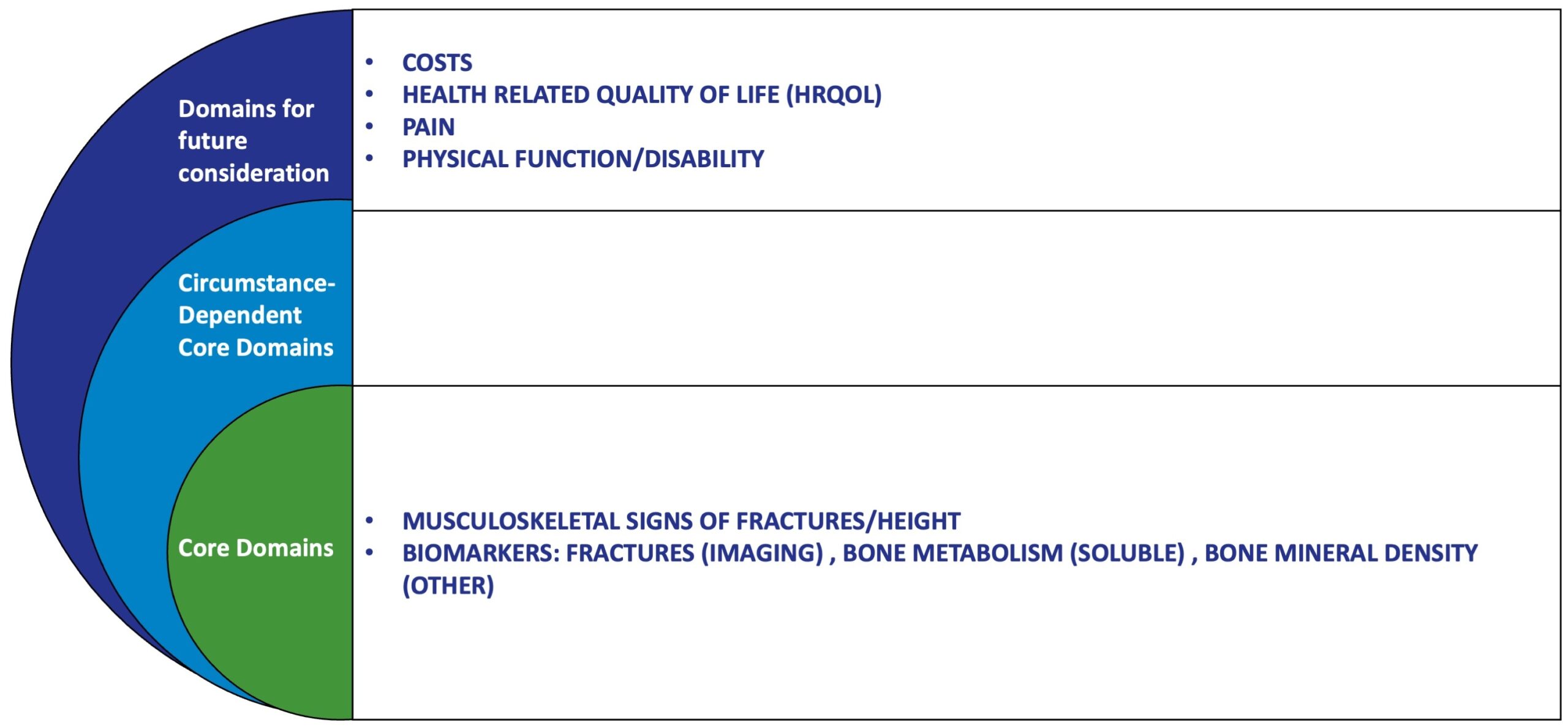
The OMERACT Osteoporosis Working Group was established to define and standardize outcome measures for randomized clinical trials (RCTs) in osteoporosis. Given the growing burden of osteoporotic fractures and the wide variability in how clinical trials measure outcomes, OMERACT’s work has been instrumental in guiding consistency, comparability, and improved evidence synthesis in this field.
At OMERACT III (1996), held in Cairns, Australia, experts in rheumatology, endocrinology, radiology, and patient-centered research came together to propose a Core Outcome Set for osteoporosis trials. These recommendations focus on outcomes that are clinically meaningful, sensitive to treatment effects, and feasible to implement across global studies.