How to cite this version of the Handbook
Beaton D, Maxwell L ,Grosskleg S, Shea B, Tugwell P (editors). The OMERACT Handbook Version 2.1 [updated April 2021]. OMERACT. Available from https://omeract.org/handbook/
To cite a specific chapter please put the chapter number and name before the list of the editors. Eg. Beaton D, Maxwell L ,Grosskleg S, Shea B, Tugwell B (editors). The OMERACT Handbook Version 2.1 [updated April 2021]. OMERACT. Available from https://omeract.org/handbook/
Reproduction and translation
Permission from the editors is required to reproduce or translate the OMERACT Handbook.
The OMERACT Handbook © 2021 by OMERACT is licensed under CC BY-ND 4.0
This handbook is a freely accessible resource open to anyone – patient research partners, clinicians, researchers, regulators, industry trial developers, trial funder, technology implementation decision-makers, or others interested in how best to develop a Core Outcome Set (COS).
Chapter 1: Purpose, Structure, and Evolution of OMERACT
Chapter 1 of the OMERACT Handbook introduces the essence of OMERACT as a leading force in core outcome set methodology, its historical emergence and evolution. It delves into the organization's structure, inclusive consensus-building approaches, and global impact, emphasizing the ongoing development of methodologies and core outcome sets, underscoring its legacy and promising future.
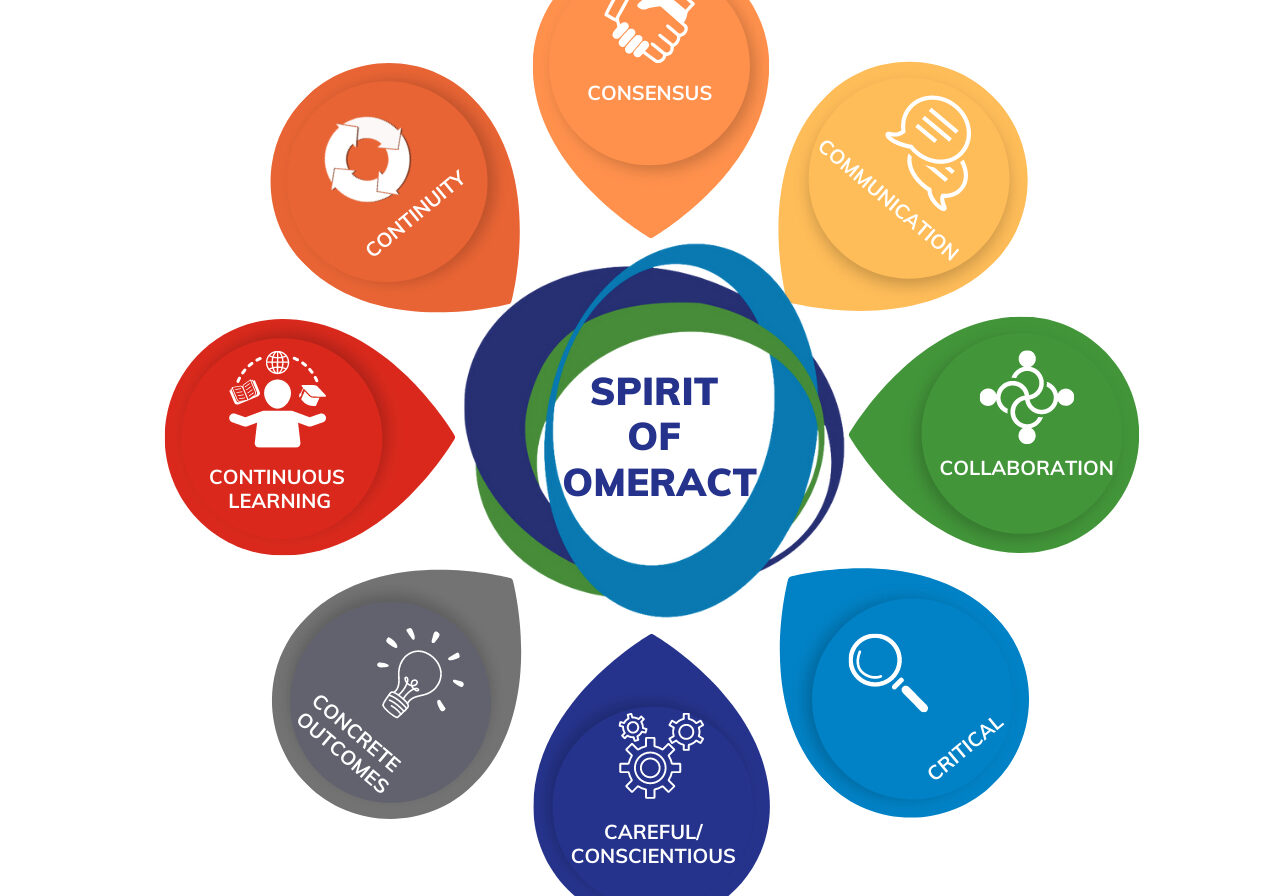
Chapter 2: Spirit of OMERACT
Chapter 2 delves into the foundational principles of OMERACT. The chapter offers a deep understanding of how OMERACT's ethos shapes its mission and operations.

Chapter 3: Pioneering Patient Research Partner Engagement and the Journey of Continual Evolution
Chapter 3 unfolds how OMERACT was the first to embrace patient research partners and continues to lead the way in this collaborative approach. This chapter delves into the ongoing evolution of patient involvement, showcasing how OMERACT's pioneering efforts have set the stage for continuous growth and improvement.
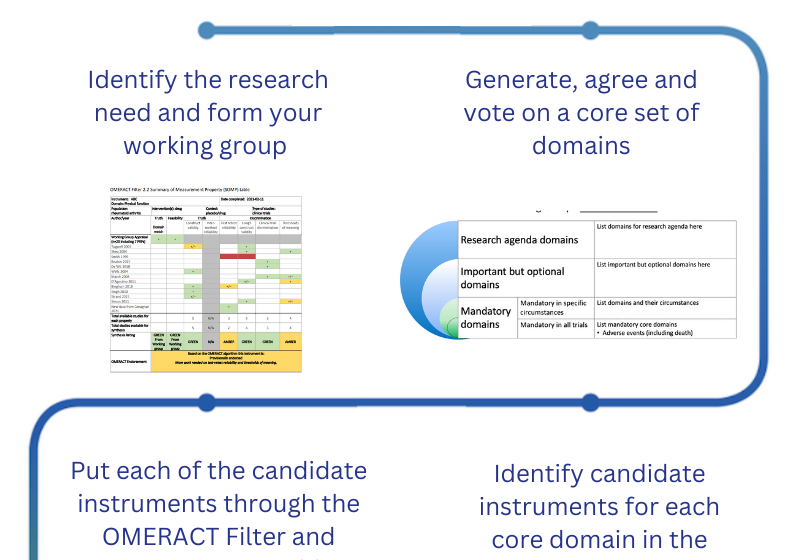
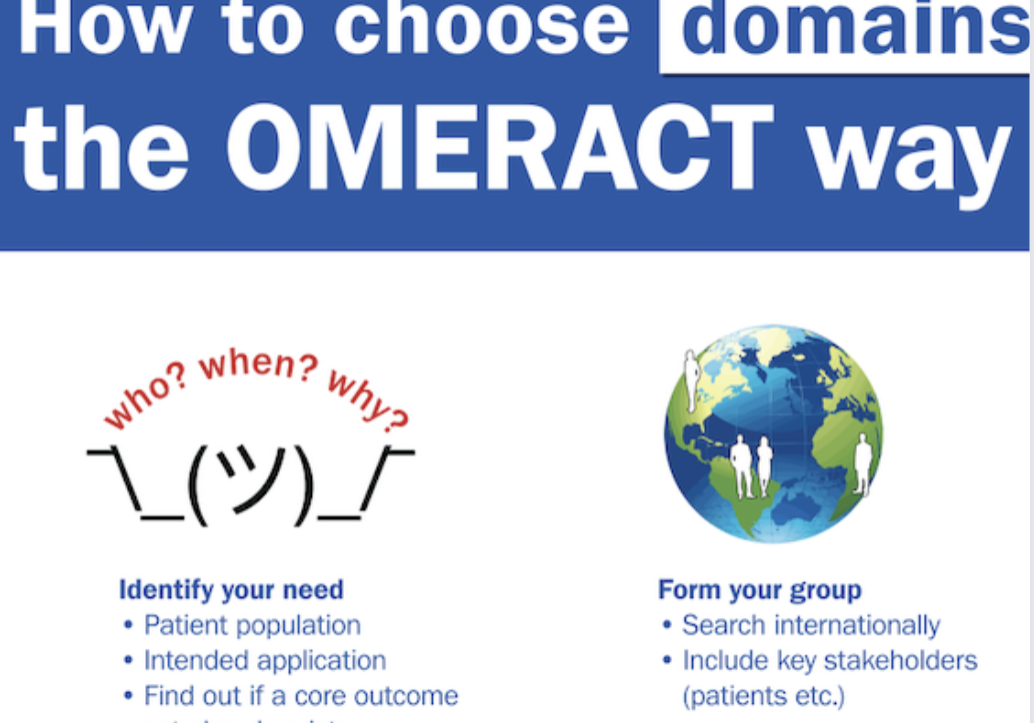
Chapter 4: Developing Core Domain Sets
Chapter 4 outlines a rigorous and transparent process for generating, agreeing and voting core domains that should be measured in clinical trials for specific health conditions across the OMERACT Core Areas.
Chapter 5: Instrument selection for Core Outcome Sets
Chapter 5 delves into the process of selecting instruments for Core Outcome Sets. This process, guided by the "OMERACT Filter," assesses instruments based on the pillars of Truth, Discrimination, and Feasibility, ensuring their suitability for inclusion in Core Outcome Sets for clinical trials.
Chapter 6 – Developing OMERACT Methodology
This chapter focuses on the OMERACT Way for Developing OMERACT Methodology. It outlines a process for either pioneering new methodologies within the OMERACT framework or refining existing ones.