About the Shoulder Disorders Working Group
Shoulder disorders, including rotator cuff disease (tendinopathy, impingement, subacromial bursitis, tears), adhesive capsulitis, instability, glenohumeral osteoarthritis, dislocation, proximal humeral or humeral head fractures, and unspecified shoulder pain, are highly prevalent disorders (7–26%), and are associated with significant morbidity, disability, and economic burden. Despite increasing numbers of trials investigating the benefits and harms of treatments for these disorders, there is as yet no widely endorsed core domain set (for outcome domains) or core outcome measurement set (for instruments) that is advocated for clinical trials. The Outcome Measures in Rheumatology (OMERACT) Shoulder Working Group was established in 2015 to develop these sets for clinical trials of shoulder disorders addressing all types of interventions.

Rachelle Buchbinder
Co-Chair

Joel Gagnier
Co-Chair

Sofia Ramiro
Co-Chair

Arianne Verhagen
Co-Chair

Sam Whittle
Co-Chair

Romi Haas
Emerging Leader

Pamela Richards
Patient Research Partner
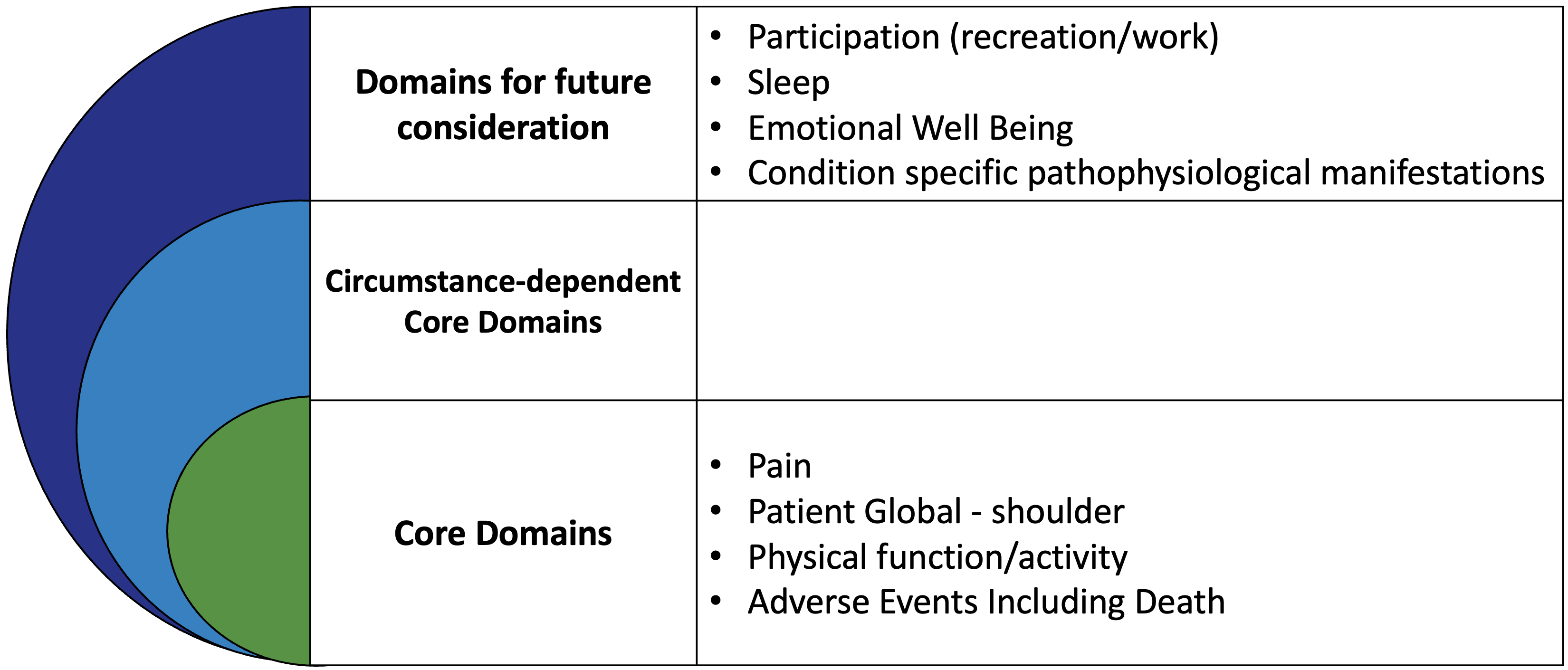
OMERACT Endorsed Core Domain Set for Shoulder Disorders
RECENT WORKING GROUP PUBLICATIONS
The OMERACT core domain set for clinical trials of shoulder disorders
Identifying a core set of outcome domains to measure in clinical trials for shoulder disorders: a modified Delphi study
Patients' experience of shoulder disorders: A systematic review of qualitative studies for the OMERACT Shoulder Core Domain Set
Outcome Reporting in Randomized Trials for Shoulder Disorders: Literature Review to Inform the Development of a Core Outcome Set
Creation of a core outcome set for clinical trials of people with shoulder pain: A study protocol